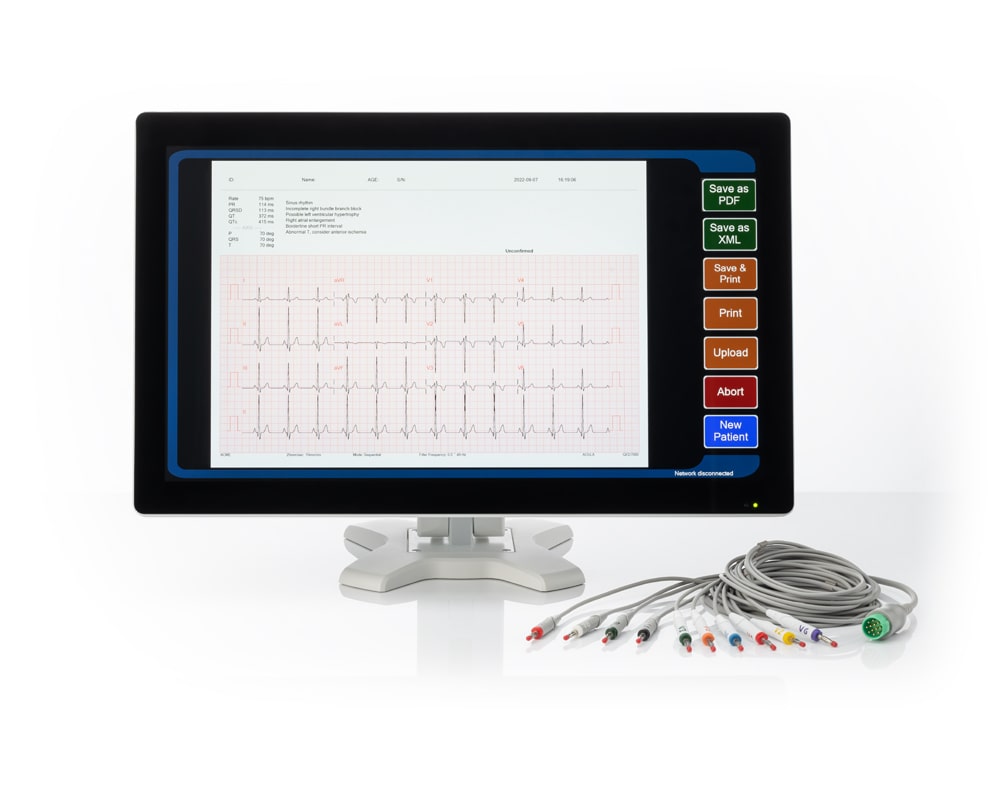
QED 3130
21.5" 12 Lead Electrocardiograph (ECG)

What is the QED 3130?
The QED 3130 is a complete 12-Lead Electrocardiograph (ECG) solution with ECG interpretation software. Designed to assist health care professionals in delivering the utmost quality care to patients, ECG signals can be easily and accurately acquired, analyzed, stored and printed in one touch. With features designed to enhance portability, streamline workflow and assist in a physician’s diagnosis, the QED 3130 12-Lead ECG is suitable for many environments.
Clinically tested and with a high sensitivity and accuracy, QED 3130's proprietary algorithm performs accurate measurements and analyzes ECG parameters to generate insightful advisory interpretations.
The QED 3130 is cleared by CE (0112) in Europe for the product category: 12 Lead ECG Recorder (certificate): click here to view certificate.
Warranty
The QED 3130 comes with a one-year limited warranty. For more information, please click here.
Clinical Studies Performed At

QED 3130 Features
21.5" Full HD water resistant touch screen allows physicians to easily view and input patient data
Tested for cybersecurity to protect personal information and exam integrity
Quick power up and shut down for zero disruptions in workflow
Twelve (12) lead patient ECG preview in Full HD
ECG interpretation software quickly assists healthcare professionals in their diagnosis
Large handle for portability and 3-step set up
Unlimited memory via external USB memory
Print from standard commercial printers on A4 or Letter sized paper
Smart features alert users when electrodes are placed improperly or not securely attached
Easily export patient ECGs to a PDF or XML report format or transfer it via DICOM



Optional Upgrade: ECG Analysis Program
Automatic 12 lead ECG interpretation program: The program is intended to provide an interpretation of the resting 12-lead ECG in all patient-care situations, whether it is a hospital setting or primary care setting. It is capable of diagnosing all commonly recognized ECG abnormalities, such as myocardial infarction (MI), including acute MI, ventricular hypertrophy, ST-T abnormalities, and common abnormalities of rhythm. Conduction defects and other abnormalities, such as prolonged QT interval, are also reported.
Non-invasive Ischemia Detector (NID): Patented, innovated technology that provides accurate screening of ischemia without risky and invasive procedures. For more information, please visit our dedicated page to NID.
| Standard Measurement |
|---|
| Heart Rate |
| QRS Duration |
| PR Interval |
| QT Interval |
| ST Segment |
| Rhythms Interpretation |
|---|
| Normal Sinus Rhythm |
| RBBB, LBBB |
| Ventricular Tachycardia |
| 1st, 2nd degree AV Block |
| Sinus Bradycardia |
| Sinus Tachycardia |
| APC, VPC |
| Acute MI |
| LAH, RAH |
Technical Specifications
| Power Supply Voltage | AC Adapter: 100-240VAC, 50-60Hz |
|---|---|
| Sampling Rate | 3000Hz/lead |
| Gain Settings | 5, 10, 20 mm/mV, ±5% |
| Timeline Selection | 25, 50 mm/s, ±5% |
| ECG Interpretations | 26 Categories |
| Heart Rate | 30 to 250 beats/min ±5bpm ±10% (the larger of the two) |
| Record Mode | PDF, XML, DICOM |
| LCD Display | 21.5" FHD (1920x1080), IP65 (front design) with PCT + AR coating |
| Data Entry | Touch screen |
| Platform | Intel® Atom e3845 1.9Ghz, 4G RAM |
| Storage | Built-in or flash drive |
| Power Supply | 12V 5A DC |
| Size | Width: 20.7" (527mm), Depth: 3.1" (79mm), Height: 12.8" (324mm) |
| Weight | 18.7lbs / 8.5kg |
| Safety & Performance | IEC 60601-1, IEC 60601-2-25 |
Request More Info
If you have any questions, please fill out the form below. We will contact you shortly. Thank you for your interest!
Please note that this product is not currently available for sale in the US.